-
Blog
-

Turkish Translation Partner for Eur...
Discover why European LSPs benefit more from a dedicated Turkish translation office than freelancers — hi...
-
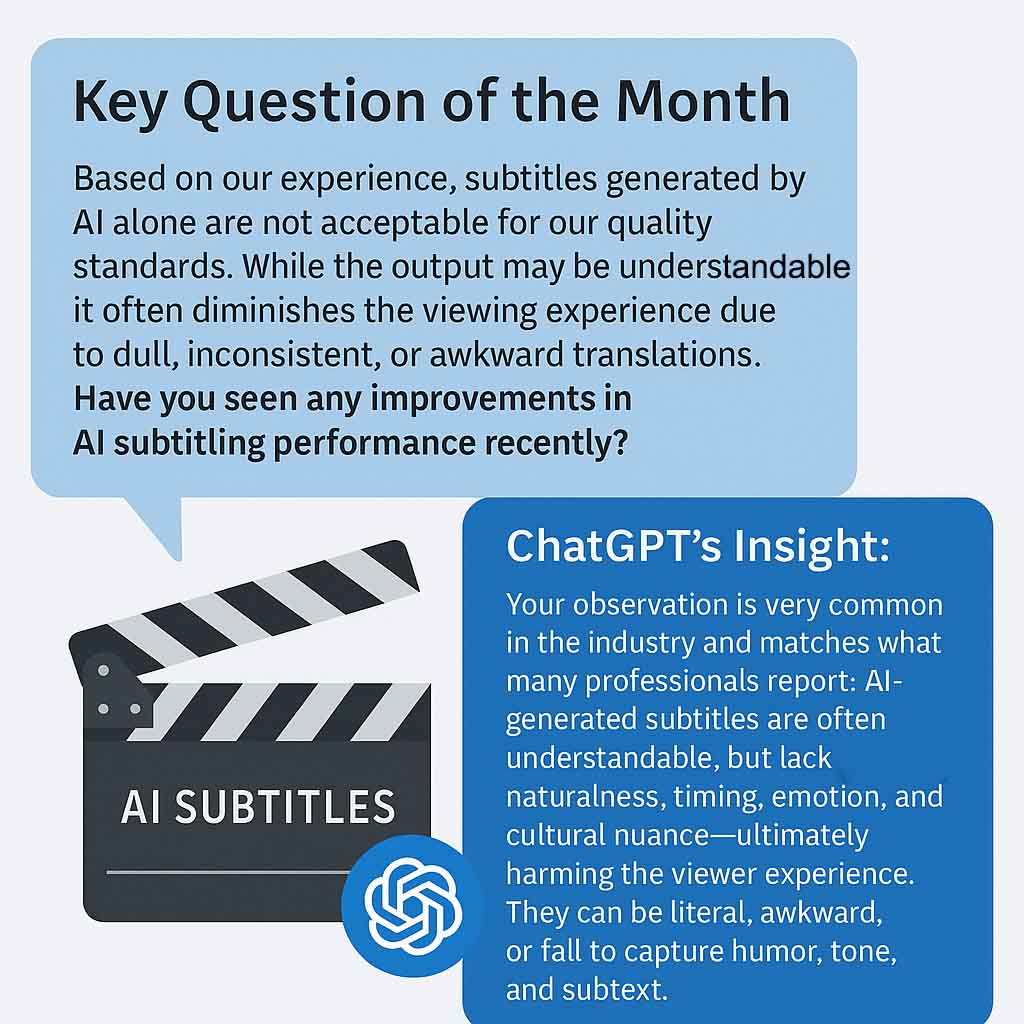
Subtitles generated by AI alone
Explore how AI-generated subtitles compare to human-edited ones—understand current limitations, improveme...
-
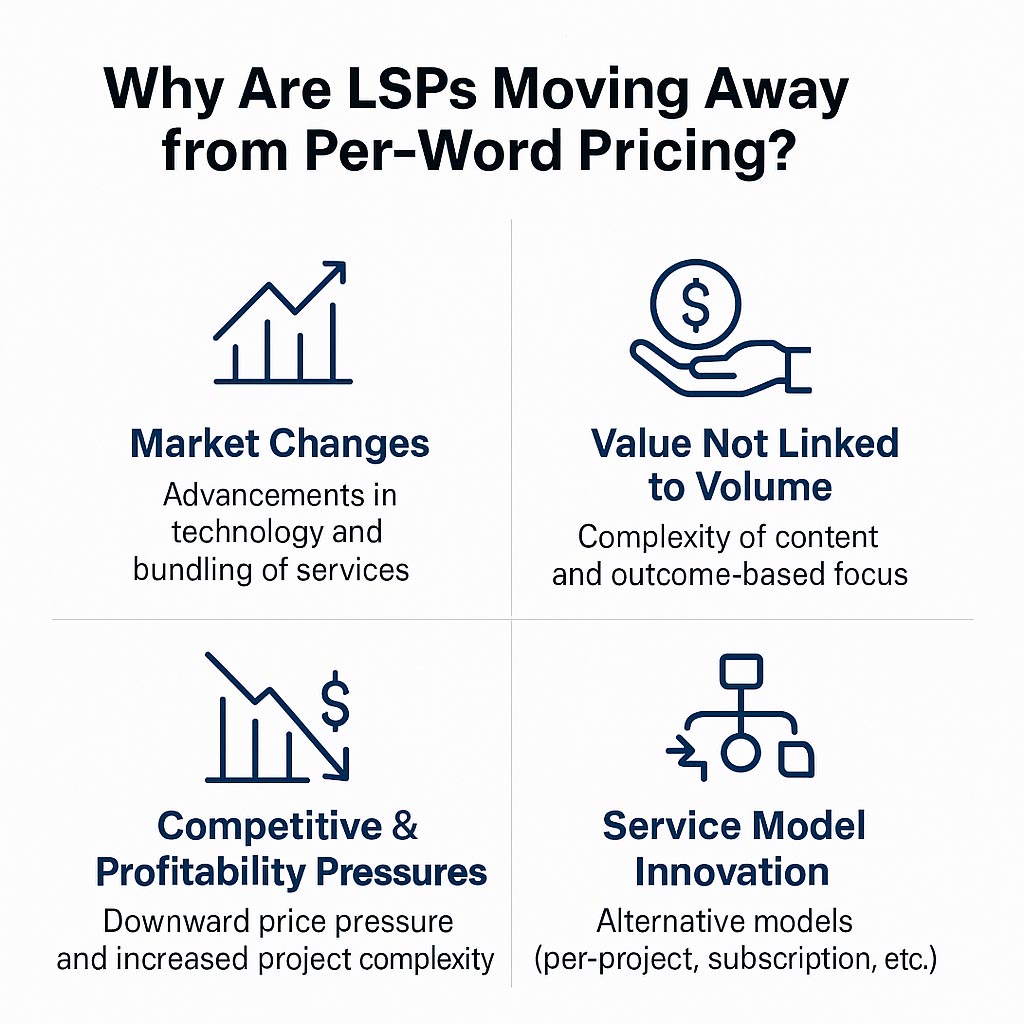
Why Are LSPs Moving Away from Per-W...
Discover why modern language service providers (LSPs) are shifting away from traditional per-word pricing...
-

AI in Media Translation: Promising,...
AI in Media Translation: Promising, But Not Yet Production-Ready
-

Characteristics of Enterprise-Level...
Discover the key characteristics of enterprise-level localization projects, including translating and loc...
-

Brief History of our Vendor Database
Discover the evolution of our Global Vendor Network, a meticulously developed platform supporting over 23...
- View All
-
-
Our Services
-

Project & Document Management
Comprehensive oversight of your translation projects for streamlined, consistent, and on-time delivery across industries
-

Terminology Management
Harmonized terminology for clear, consistent, and professional translations, ensuring your message is always on point
-

Quality Management
Robust quality assurance built on ISO standards and best practices for translations you can trust
-

Al-powered Enterprise Solutions
Flexible, multilingual support tailored to your company’s growth, with scalable teams and streamlined processes
-

Translation by Specialists
A professional, industry-specialized translators

Web / Apps / Games Localizations
Culturally adapt your content across global markets

Interpretation
Skilled Turkish interpreters for your events
Al-powered translation
AI translations refined by human experts

Media Translations
Turkish subtitling and media localization

Transcription
Accurate Turkish transcription services

Desktop Publishing
Cost-efficient DTP for quality output

Technology
Technology-Driven Linguistic Consulting & Infrastructure
-
-
Industries
-

Translation Services for Every Industry
Which Industries Most Rely Upon Language and Translation Services
-

Industrial Manufacturers
Accurate translations of technical manuals, product specifications, engineering documents, and compl... -

E-commerce and Retail
User manuals, product descriptions, FAQs, troubleshooting guides, and safety instructions tailored f... -

Technology and IT industry
Specialized translations for technology and IT research, industrial studies, and scientific publicat... -

Media
Translations for digital, broadcast, interactive, and corporate media, covering all communication an... -

Business laws and contracts
Precise translations of contracts, agreements, bylaws, legal opinions, court decisions, and regulato... -

Hospitality
Tour and hotel guides, travel documents, restaurant menus, online selling platforms, and customer fe... -

Your Industry
We also cover other specialized fields. Let us know your unique needs, and we’ll provide tailored su... -

Government & NGO
Translations for tenders, technical and administrative documents, environmental policies, social sci... -

Financial
Financial statements, banking documents, insurance policies, stock market analysis, and credit lette... -

Healthcare
Specialized translations for clinical trials, pharmaceutical guidelines, medical devices, and health...
-
-
Get Quotes
-

Instant Quote
Get a quick, no-obligation quote for translation or language services by filling out this form and sharing your source files or samples with any special instructions
-

Multilanguage Quote - Enterprise
For complex or multilingual projects, use this form to share extra details and files (references, glossaries, translation memory) for a tailored quote
-

Quote for Interpreters - Conference, Panel, Meeting
To book professional interpreters for your event, fill out this form with details like languages, venue, and interpreting type to help us provide a quick, accurate quote
-
- Contact Us
-
Vendors
-
 English
English










